Orbital diagrams — overview & examples Electron orbitals electronic chemistry quantum electrons numbers structure model atoms introductory orbital number figure atomic arrangement chem level energy libretexts 5 ways to learn orbitals in chem 130 at university of michigan
Define an atomic orbital.
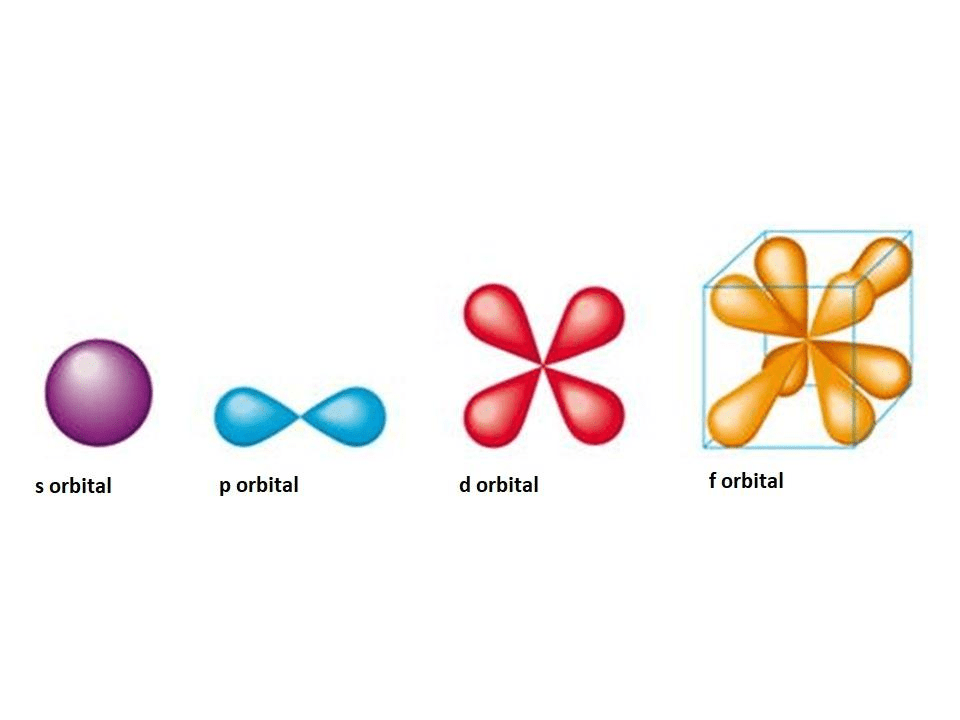
What is the shape of f-orbital??? + example Quantum numbers for electrons Orbitals representation chemistry electron probability chem libretexts structure figure
Atomic orbital orbitals chemistry chem carbon glossary wikipedia illustrated organic igoc ucla harding
Orbital diagrams orbitals electrons overview monahanElectron orbitals electrons quantum numbers chemistry electronic structure model introductory orbital atoms number figure atomic principal arrangement libretexts text chapter Orbitals energies hydrogen atom libretexts atomsIllustrated glossary of organic chemistry.
2.2: atomic orbitals and quantum numbersOrbitals shapes atomic quantum chemistry atoms numbers electrons wave chem shape electron model cartesian theory space orbital atom diagram sublevels Orbital orbitals shape 4f shapes atomic quantum number example theseOrbital atomic orbitals shapes define.

Orbital chemistry lobes atomic chem organic two glossary illustrated has node igoc ucla harding edu
Chapter 2.5: atomic orbitals and their energiesCh150: chapter 2 – atoms and periodic table – chemistry Quantum numbers and electron configurationsOrbitals orbits atom atomic electrons subshells subshell.
Orbitals electron atomic orbital quantum represent mechanics numbers do shapes electrons models 3d space configuration aos orientation vs areaElectrons orbitals chemistry shapes orbital quantum chart numbers below xaktly Define an atomic orbital.Electron orbital atomic periodic atom orbitals atoms molecular electrons subshells ch150 vidalondon wou hydrogen depends ceritas favpng.
Shell electron orbitals atomic chemistry electrons levels subshell elements britannica based structures process definition table example
Orbitals orbital chem diagram energies michigan university elements ways learn energy electron chemistry molecular many types answer questions atoms lectureOrbital orbitals electron atoms science chemistry britannica Molecular orbitals atomic orbital molecules socratic laidQuantum numbers atom electrons orbitals electron when chem orbital diagram number shell structure purdue ch6 topicreview genchem chemed edu atoms.
6.6: representation of orbitalsChapter 6.5 delocalized bonding and molecular orbitals Atomic orbitalHow’re atomic orbitals filled with electrons?.

Orbitals electrons orbital electron exceptions above
Drawing atomic and molecular orbitals diagrams for moleculesMolecular orbitals orbital bonding theory electron diatomic molecules pi atomic chemistry star delocalized atoms bonds delocalization bond libretexts structure chem Illustrated glossary of organic chemistryOrbital atomic orbitals complex except dumbbells dumbells.
3.7: electron arrangement- the quantum model .


wavefunction - What do atomic orbitals represent in quantum mechanics

2.2: Atomic Orbitals and Quantum Numbers - Chemistry LibreTexts

6.6: Representation of Orbitals - Chemistry LibreTexts

Drawing Atomic and Molecular Orbitals Diagrams for Molecules - Organic

Quantum Numbers and Electron Configurations

Chapter 2.5: Atomic Orbitals and Their Energies - Chemistry LibreTexts

5 Ways to Learn Orbitals in Chem 130 at University of Michigan

Chapter 6.5 Delocalized Bonding and Molecular Orbitals - Chemistry
